You are 18 years or older

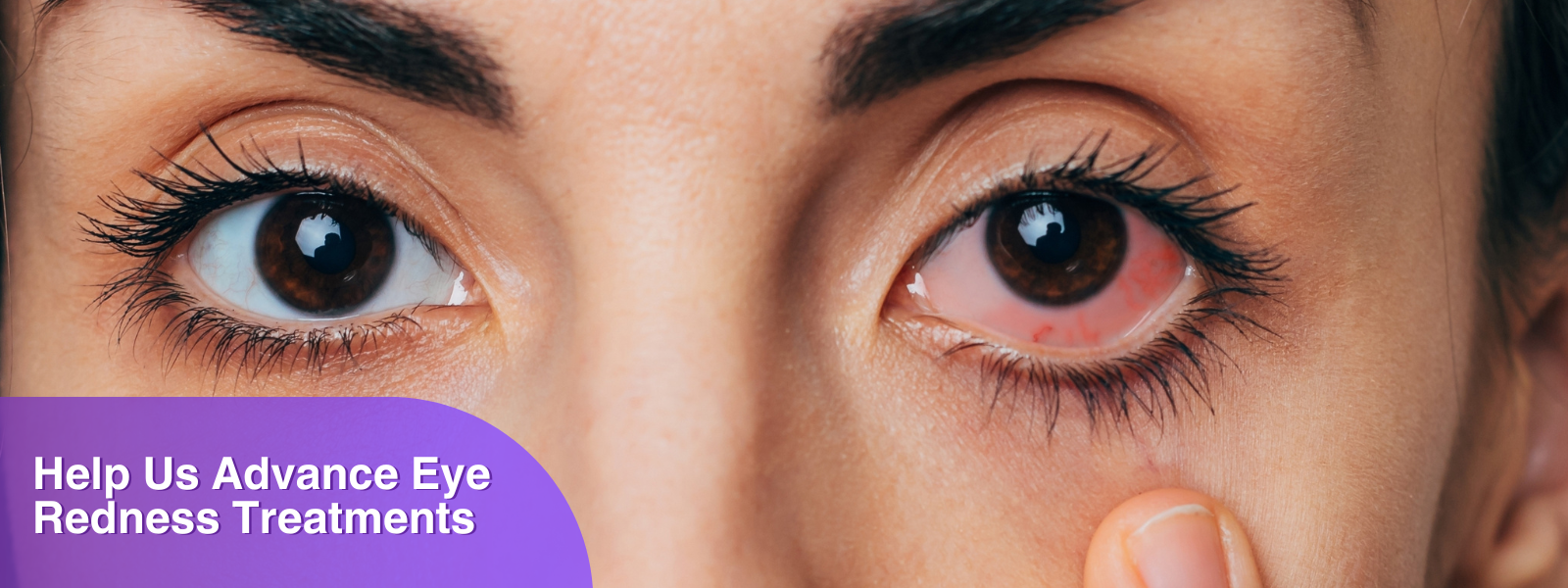
You have been experiencing chronic ocular redness
Have used or desire to use eye drops for eye redness in the last 6 months
You have not undergone eye surgery in the last three (3) months or refractive surgery (LASIK) in the last six (6) months